Who are we?
CLARIPURE® – are a team of biotechnology professionals utilizing extremely high-grade polymeric products, providing cost-effective solutions for all your single-use bioprocessing requirements, from laboratory to production scale – and everything in between, both upstream & downstream, incorporating cell culture & Separation, Media & Buffer Prep & Harvest, through to Purification & Final fill – and operating out of a global network of ISO 14644 Class VII Cleanrooms and self-contained laboratories. Working with the industry leaders in their field, for tubing, connectors, sensors, filters & Bioprocessing Bags (BPBs) – CLARIPURE® are able to provide the Life Sciences industry with novel Single- Use bioprocessing equipment and systems; cell-culture testing & production capabilities; as well as bioprocessing software & training services.
CLARIPURE® are a subsidiary of the NEUMO Ehrenberg Group, a diverse global group of 34 engineering & manufacturing companies with over 2000 employees worldwide, with a global revenue of US$700m. Our heritage dates back to 1947, supplying the BioPharma & Life Sciences and the Food & Dairy industries for 7 decades with innovative and patented sterile technology products…engineering excellence is in our DNA.
All of the solutions provided by CLARIPURE® for your Single-Use bioprocessing requirements – systems & components – are USFDA, USP Class VI & EU Pharmacopeia approved, and designated ADCF/ADIF/ADI(Animal Origin Free)
Using only the highest grade polymeric materials, our holistic approach to your critical fluid procedures, ensure both the integrity & sterility of your systems & fluid process flows.

- USP Class VI, USFDA & EU Pharmacopeia certified & approved materials
- Global network of ISO 14644 Class VII Clean Room facilities
- cGMP Compliant materials & facilities
- Sterility validation available to ISO 11137 (SAL 10-6)
- All products classified ADCF/ADIF/ADI (Animal Free Origin)
- ISO 9001 Certified
- Heritage of innovative & patented sterile technology
- Holistic fluid logistics approach to ensure sterility
- Experience, Innovation, Quality & Supply chain management in Bioprocessing
- Heritage of innovative & patented sterile technology
- Holistic fluid logistics approach to ensure sterility and cleanability
- Full range of branded quality flow component products
- Innovation, Quality & Supply chain management in BioProcessing
- Same approach with Hi-Grade Polymer Single-Use Technology
- ISO 14644 Class 7 Clean room facilities
- All products USP Class VI & FDA Approved
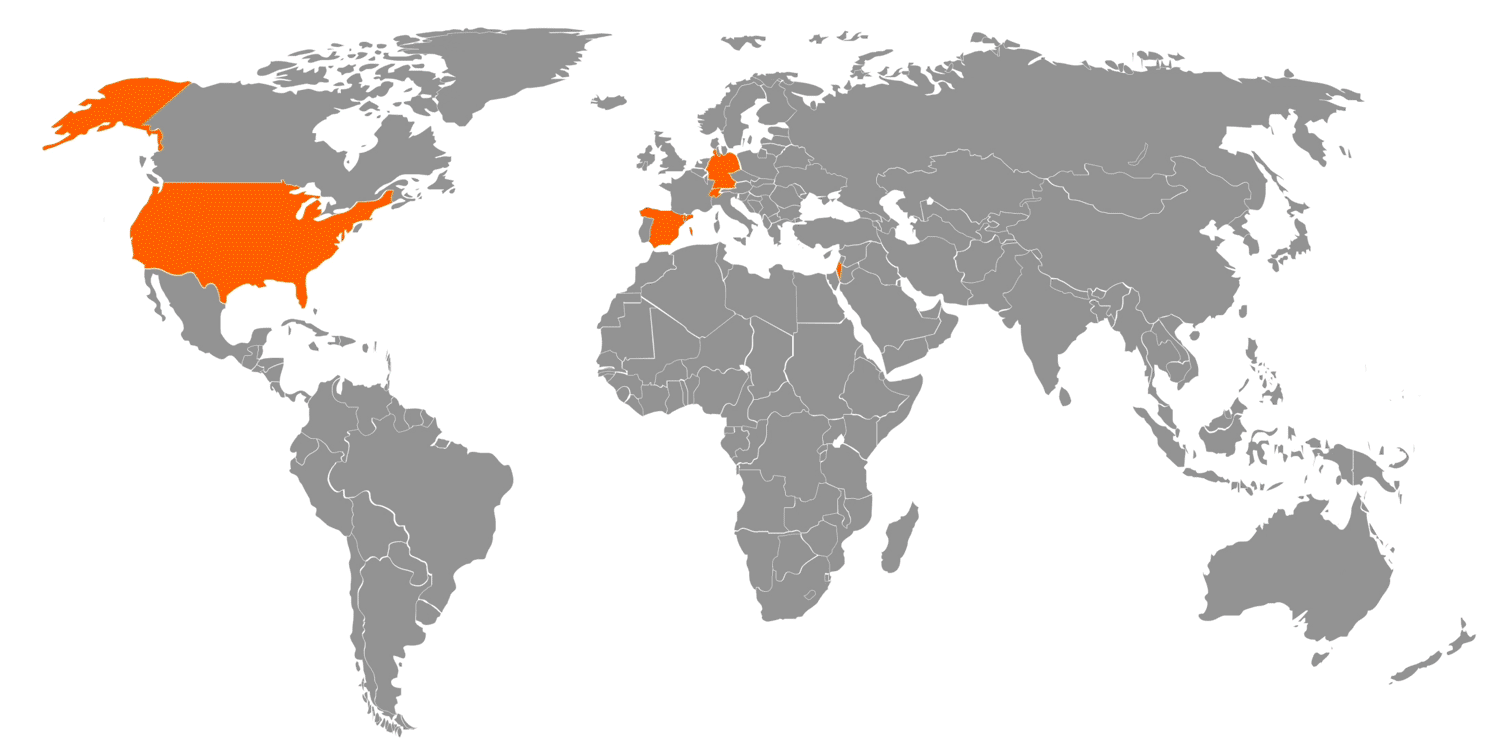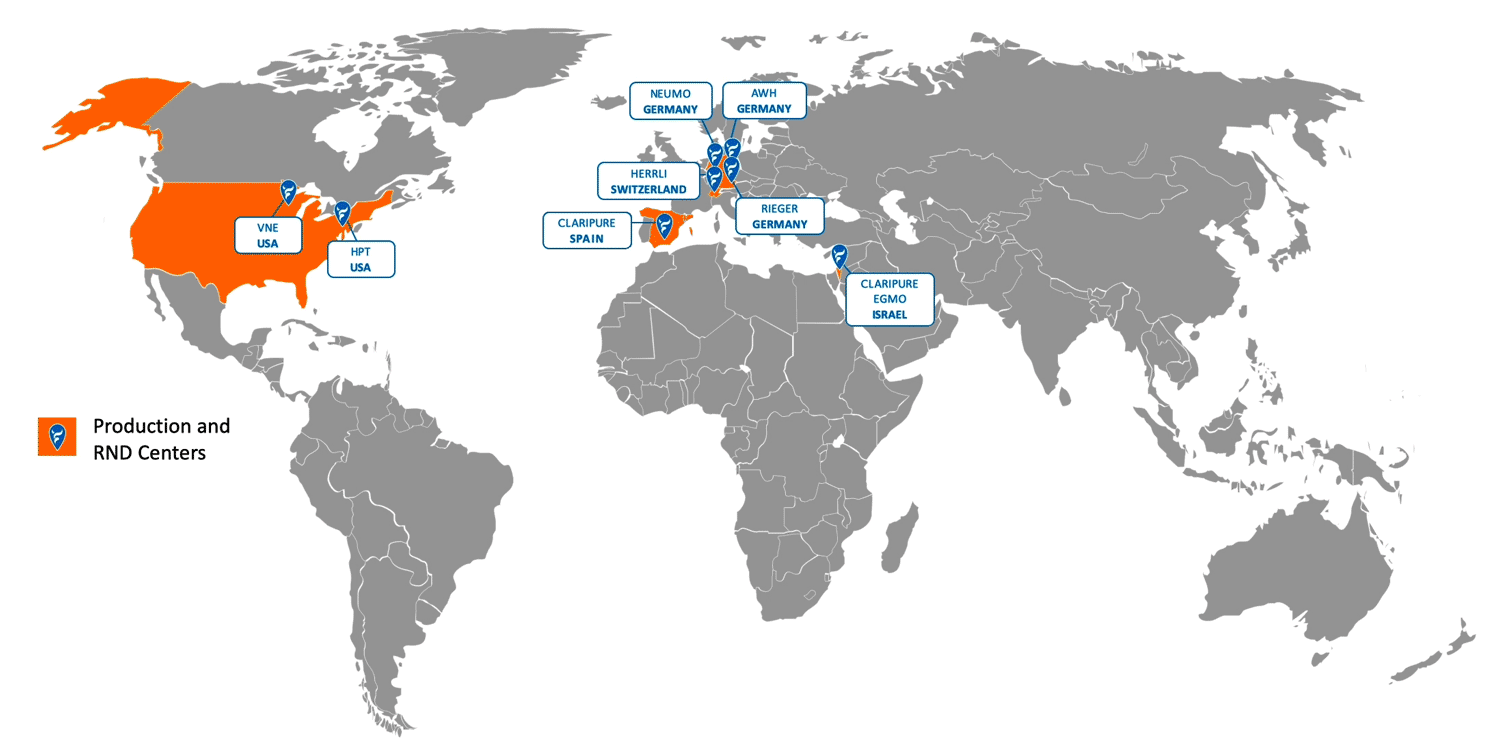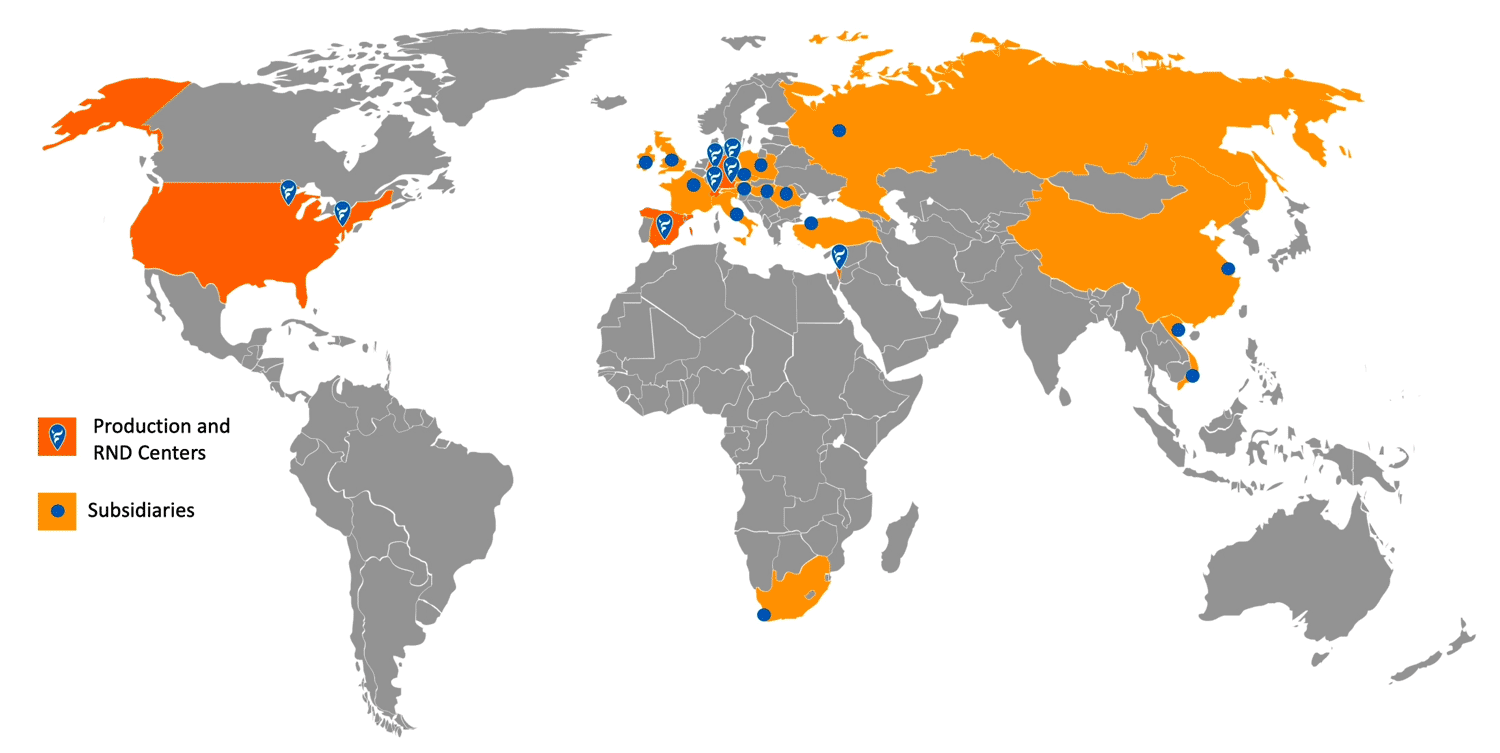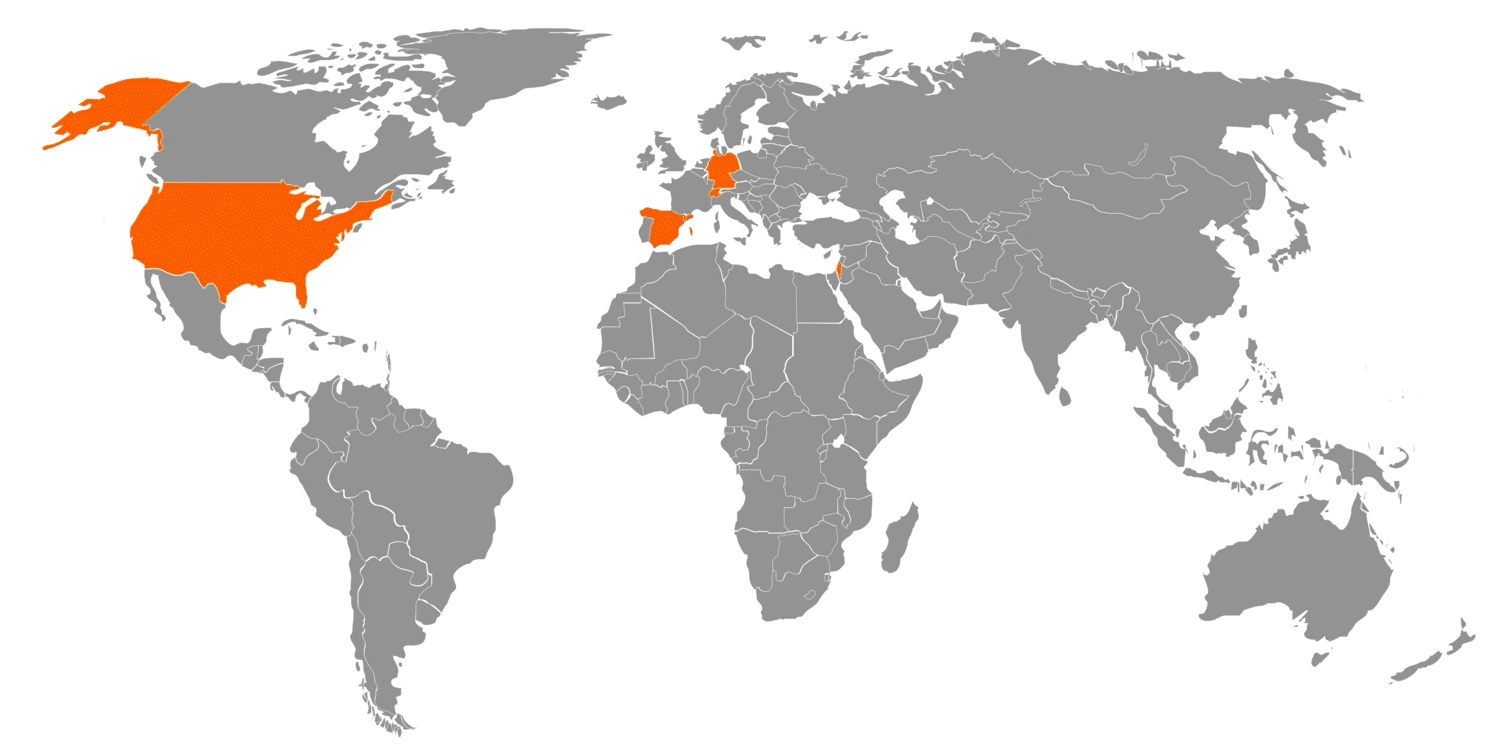
NEUMO Ehrenberg Group – Global Footprint:
0
Decades of quality
and dedication
and dedication
0
Companies in a muntilational organization
0
Divisions
0
Employees
0
Million US$
annual sales
annual sales
Our clients are our best reference



